|
|
Post by ckfan on May 19, 2017 13:51:28 GMT
Superb work birkie. Absolutely amazing. Over my head for sure but you did great work. I'm glad to hear that keyboard duster is a good replacement for SO2. Even better than we originally thought!
|
|
|
|
Post by birkie on May 19, 2017 14:08:33 GMT
Superb work birkie. Absolutely amazing. Over my head for sure but you did great work. I'm glad to hear that keyboard duster is a good replacement for SO2. Even better than we originally thought! Yeah, I mainly just wanted to see if theory could explain what we've been seeing. However, as the great Yogi Berra may or may not have said:"In theory, there's no difference between theory and practice. In practice, there is". Observing how things actually behave in the real world is far more valuable, especially with regard to practical details such as susceptibility to oil logging, accumulation of refrigerant in the dome, etc. But at least it backs up what the wise pioneers/practitioners like coldspaces have been seeing so far! |
|
|
|
Post by ckfan on May 19, 2017 18:57:57 GMT
You are right. Nothing ever behaves the way you think it will. After watching Gill add 152a to the DRF4 I can honestly conclude that there are way too many factors to consider. It seems like 152a wants to hide in the dome when you first add it in.
|
|
|
|
Post by Travis on May 19, 2017 22:32:13 GMT
Just to answer the question above, I haven't got the DRF4 back from the painter. I was told it would be blasted today.
|
|
|
|
Post by birkie on May 20, 2017 2:56:56 GMT
Well, I tried doing some calculations to model the effects of suction line restriction. I'm still not sure I have the thermodynamics completely right, but the models suggest that suction line restriction raises the discharge temperature, and lowers the efficiency. I guess that makes sense. Compared to shaving the piston you still have the same mass flow (nothing changes in the evaporator, it's still boiling at the same rate under the same conditions), but now the compressor is pumping against a higher pressure difference due to the throttling from the restrictor.
Flow-restricted power draws (normalized for capacity), relative to SO2:
- R134a: 1.24
- R152a: 1.21
- R227: 0.92
- R1234ze: 1.03
- R12: 1.28
- R1234yf: 1.25
So most of them would theoretically draw a bit over 20% *more* power than SO2 when using a restrictor, at identical capacities. R227 is the most efficient when throttled, but still has has a very high mass flow. R-1234ze stands out as just a mere 2-3% less efficient than SO2, with a mass flow just slightly worse than R134a. Theoretical discharge temps of R152a (the highest of the bunch) are about 150F, still considerably cooler than SO2.
Given how little power these refrigerators consume, the differences are really just academic, but fun to think about.
|
|
|
|
Post by birkie on May 20, 2017 3:11:01 GMT
By the way, I've never seen the insides of a DR or a CK in real life, but would their internal construction allow a metal "head gasket" to be inserted? That might be a less destructive method of reducing the volumetric efficiency to match some of the modern refrigerants, compared to machining the piston.
|
|
|
|
Post by ckfan on May 20, 2017 21:09:13 GMT
I don't think the DR has a head gasket but I believe the CK does. Or at least that is how I'm remembering it.
|
|
|
|
Post by ChrisJ on May 21, 2017 3:42:42 GMT
Birkie,
Amazing, thank you!
Would love to see something similar done for methyl formate (R611) vs R11 and R123 as well as any other low pressure refrigerants.
I'm a bit surprised, I always thought SO2 was considered a very good refrigerant even now.
|
|
|
|
Post by birkie on May 21, 2017 13:48:03 GMT
Sure, I can crunch some numbers for low-pressure refrigerants. R-1233dz (an HFO) looks like it might be interesting to throw in the mix as well. It's an A1 refrigerant being considered as non-toxic, low ODP and GWP replacement for R123. The problem is that I can't find an equation of state for Methyl Formate! It should be possible to compare mass flow and get a pretty good estimate for normalizing for capacity, but I wouldn't be able to compare power consumption of the various refrigerants to methyl formate; just to each other. As far as SO2, the best things it has going for it are its low mass flow, and low condensing pressures, and low head. |
|
|
|
Post by birkie on May 21, 2017 14:20:53 GMT
I don't think the DR has a head gasket but I believe the CK does. Or at least that is how I'm remembering it. OK, this pic shows it clearly on a DR. It looks like all the sealing action takes place at the valve seat. There's also that funny little oil passage. Hmm.  |
|
|
|
Post by ChrisJ on May 21, 2017 23:04:12 GMT
Not sure if this helps? Attachments:
|
|
|
|
Post by coldspaces on May 22, 2017 1:16:13 GMT
Oh interesting, the thought of blends hadn't even crossed my mind! There doesn't seem to be much literature out there on HFO blends yet, at least that I could find, in the pressure ranges we're looking for. For what it's worth, it looks like HFO1234yf forms an azeotropic mixture with R134a, forming a non-flammable (A1) low-GWP blend at about 10% R134a: bioage.typepad.com/files/R1234yf_R134a_Heat%2520pump.pdf. I'm curious to see if/how CoolProp models mixtures, it'd be neat to try it out tonight and see what happens. I don't think it would be able to predict if a mixture is azeotropoc vs zeotropic, though. I'm assuming we'd need a mixture to be azeotropic, to avoid accumulating one of the components in the evaporator over time? After reading this Ray and I did some playing in the garage today. Evacuated a 30 lb recover tank and put 10oz of 152a in it. We then added 3 oz 134a for a bout a 77/23 blend. Mind you in that much volume it was not enough pressure to make either refrigerant condense. This made it a bit unsure just how well the two were mixing. I then did a flame test on the mix and it was still flammable. We then added 2 more oz 134a and assuming the little I let out for the test didn't lose much 152a we now had about a 67/33 blend at this point it is still flammable but not as bad. We then added 5 more oz 134a to get about a 50/50 blend. At this point it was still a bit flammable but not near as much as when we started. From this very scientific study we concluded that too much 134a is needed to cut the flammability of the 152a and would most likely mean a blend that was higher presser then would work in place of so2 |
|
|
|
Post by birkie on May 22, 2017 13:34:57 GMT
From this very scientific study we concluded that too much 134a is needed to cut the flammability of the 152a and would most likely mean a blend that was higher presser then would work in place of so2 Fantastic experiment, but too bad that R134a didn't tame the R152a! Doing a bit of digging, there's an article that does a great job of illustrating what's going on with R152a. www.kth.se/en/itm/inst/energiteknik/forskning/ett/projekt/koldmedier-med-lag-gwp/low-gwp-news/nagot-om-koldmediers-brannbarhet-1.575938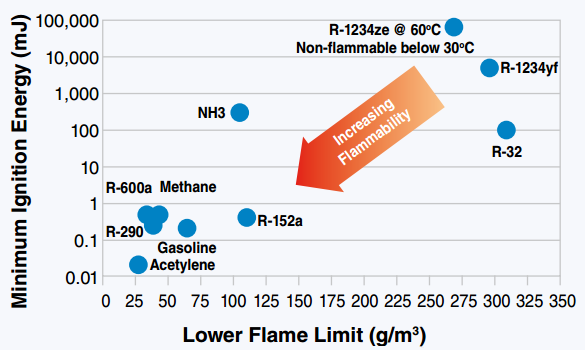 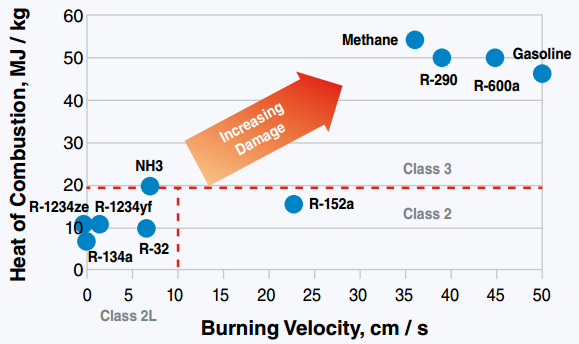 So R152a has a very low ignition energy, i.e. could possibly be ignited by a strong spark, unlike R1234yf where you'd basically need a flame. It also has a disappointingly high burning velocity, though not quite explosive. I think your experiment shows that the best we can hope to realistically achieve in taming R152a by mixing it with a "good" refrigerant is to cut it down to something like an A2L. Make it harder to ignite and burn slower, but not eliminate flammability completely. That being said, your experiment also inspired me to dig even deeper in the literature. Apparently, somebody has looked into mixtures of R152a and R1234ze: journals.sagepub.com/doi/pdf/10.1177/1687814016676945They form a near-azeotropic mixture (yay!), and also cuts down flammability (probably a little worse than 134a, though). The mass flow might still be too high, however. It looks like CoolProp can model some mixtures, so once I figure out how to do that I'll post the theoretical results. |
|
|
|
Post by birkie on May 24, 2017 1:36:58 GMT
Well, I tried modeling R152a and R1234ze to see what would happen. There are all sorts of warnings in CoolProp that its treatment of mixtures is simplistic and incomplete. The results are a little uninspiring, it looks like the mixture emphasizes the negative characteristic of each one. The mixture gets real dense fast. I was hoping a dash of 152a would add a light spiciness to the mix.
Anyway, 25% R152a + 75% R1234ze seemed like the best mixture. It was about 15% less dense than straight R1234ZE, for a total of 2.5x the mass flow of SO2, un-restricted. Capacity goes up 5% more compared to straight R1234ze. Normalizing for capacity, (i.e. restricting the mass flow) there's about 10% less mass flow than straight R1234ze, or 1.85x SO2. That's a hair better R134a, but hardly worth the effort. Oh well.
Now to see if I can do an analysis of Methyl Formate and replacements for ChrisJ. Since CoolProp doesn't have methyl formate, we'll see how long it takes to do it with pencil and paper!
|
|
|
|
Post by coldspaces on May 24, 2017 2:15:31 GMT
I was thinking of mixing R123ZD and R-1234yf.
|
|